Скачать с ютуб Immunoglobulin ( Antibody) IgG, IgM, IgE, IgD, IgA in Hindi ( एंटीबॉडी और इसके प्रकार) в хорошем качестве
Скачать бесплатно Immunoglobulin ( Antibody) IgG, IgM, IgE, IgD, IgA in Hindi ( एंटीबॉडी और इसके प्रकार) в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Immunoglobulin ( Antibody) IgG, IgM, IgE, IgD, IgA in Hindi ( एंटीबॉडी और इसके प्रकार) или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Immunoglobulin ( Antibody) IgG, IgM, IgE, IgD, IgA in Hindi ( एंटीबॉडी और इसके प्रकार) в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Immunoglobulin ( Antibody) IgG, IgM, IgE, IgD, IgA in Hindi ( एंटीबॉडी और इसके प्रकार)
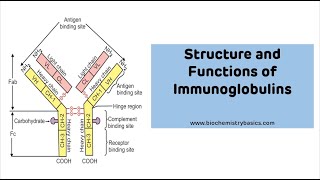
#immunoglobulins #Antibody #IgG #medical_lecture #dr_najeeb Continuing Education Activity Immunoglobulins (Ig) or antibodies are glycoproteins that are produced by plasma cells. B cells are instructed by specific immunogens, for, example, bacterial proteins, to differentiate into plasma cells, which are protein-making cells that participate in humoral immune responses against bacteria, viruses, fungi, parasites, cellular antigens, chemicals, and synthetic substances. The immunogen or antigen reacts with a B-cell receptor (BCR) on the cell surface of B lymphocytes, and a signal is produced that directs the activation of transcription factors to stimulate the synthesis of antibodies, which are highly specific for the immunogen that stimulated the B cell. Furthermore in tissue fluids and serum. This activity describes the physiology and pathophysiology of immunoglobulins. Describe theIntroduction about 20% of the protein in plasma. The immunogen or antigen reacts with a B-cell receptor (BCR) on the cell surface of B lymphocytes, and a signal is produced that directs the activation of transcription and serum. The following are 5 types of immunoglobulins in humans: IgM IgG IgA IgE IgD Function Basic immunoglobulin Structure and Function Antibodies or immunoglobulins have two light chains and two heavy chains in a light-heavy-heavy-light structure arrangement. The heavy chains differ among classes. They have one Fc region that mediates biological functions (e.g., the prevention of attachment of the microbes to mucosal surfaces, and neutralization of toxins and viruses.[1] Immunoglobulin M IgM has a molecular weight of 970 Kd and an average serum concentration of 1.5 mg/ml. It is mainly produced in the primary immune response to infectious agents or antigens. It is a pentamer and activates the classical pathway of the complement system. IgM is regarded as a potent agglutinin (e.g., anti-A and anti-B isoagglutinin present in type B and type A blood respectively) and a monomer of IgM is used as a B cell receptor (BCR).[2] Immunoglobulin G IgG is a monomer with an approximate molecular weight of 146 Kd and a serum concentration of 9.0 mg/mL. IgG is said to be divalent i-e it has two identical antigen-binding sites that comprise 2 L chains and 2 H chains joined by disulfide bonds. IgG is synthesized mostly in the secondary immune response to pathogens. IgG can activate the classical pathway of the complemen system, and it also is highly protective. The four subclasses of IgG include IgG1, IgG2, IgG3, and IgG4. IgG1 is around 65% of the total IgG. IgG2 forms an present in newborns. Immunoglobulin A IgA appears in 2 different molecular structures: monomeric (serum) and dimeric structure (secretory). The serum IgA has a molecular weight of 160 Kd and a serum concentration of 3 mg/mL. Secretory IgA (sIgA) has a molecular weight of It appears in mucosa membranes as a dimer ( activates the alternative pathway of activation of the complement system.[4] Immunoglobulin E IgE is a monomer. It has a molecular weight of 188 Kd and a serum concentration of 0.00005 mg/mL. It protects against parasites and also binds to high-affinity receptors on mast cells and basophils causing allergic reactions.[5][6][7][5] IgE is regarded as the most important host defense against different parasitic infections which include Strongyloides stercoralis, Trichinella spiralis, Ascaris lumbricoides, and hookworms Necator americanus and Ancylostoma duodenale. Immunoglobulin D IgD is a monomer with a molecular weight of 184 Kd. IgD is present in a meager amount in the serum (0.03 mg/mL) and has an unknown function against pathogens. It is regarded as a BCR.[8] IgD may play an essential role in antigen-triggered lymphocyte differentiation.[9] Receptors fo Laboratory Assessment of Immunoglobulins The quantification of immunoglobulins and the study of their functions are vital for the immunodiagnosis of immunodeficiencies, autoimmunity, hypersensitivity reactions, and inflammatory disorders. The following examinations are routinely performed for the study of the behavior of antibodies[14]: Quantitative serum immunoglobulins (classes and subclasses) IgG IgM IgA IgE IgG antibodies (post-immunization) Tetanus toxoid Diphtheria toxoid Pneumococcal polysaccharide Polio This assay evaluates the quality of the immune response after vaccination. In healthy individuals, there is at least a 1:16 titer of antibody. IgG antibodies (post-exposure) Measles Varicella-Zoster This test is to evaluate the production of antibodies against antigens after the infectious disease has occurred. Detection of isohemagglutinins (IgM) Anti-type A blood Anti-type B blood Isohemagglutinins are IgM antibodies produced by the immune system in response to bacterial antigens present in the digestive system. It has been shown that their titers may be below 1:4 in antibody deficiency disorders.